Learning on the fly: Unpacking intellectual disability genes
Genetic information is organized into a packed coil called chromatin. Chromatin remodelers attach to DNA and re-organizes how the genetic information is packaged, without changing the information contained in the DNA sequence. Seen above is the chromatin remodeler SWI/SNF interacting with a strand of DNA, referenced from the Protein Data Bank https://www.rcsb.org/structure/6TDA. Image © Kelly Bullock Art, 2020.
Reflect for a moment on how much you have learned and unlearned throughout your life. On how much your thinking has evolved since you were a toddler. This is in part due to the critical and complex development of the brain. However, problems or mistakes that occur during this key process can lead to diseases such as intellectual disability. Intellectual disability is a developmental brain disorder that is mainly characterized by deficits in cognitive functioning, including processes like thinking and memory. Even though we know the hereditary and genetic origins of some of these disabilities, we still don’t exactly understand how they lead to the disease. That is why there is a need for models to help us understand how genes control brain development and affect cognition.
Dr. Jamie Kramer’s group from Western University is studying the connection between genes and development using the fruit fly model. The team published an article in 2019, where they identified specific genes that are commonly altered or deleted in intellectual disabilities.
Nick Raun is among the collaborators of this research. I met Nick a couple of years ago at my first national conference where he told me about his work in Dr. Jamie Kramer’s lab. I was very new to the fields of genetics and neuroscience, so my interest grew as we talked about genes, intellectual disabilities, memory and brain development, since those are related to my studies as well. However, Nick mentioned that all his experiments were done in flies. I was shocked, I’d never given any thought to the brain of a fly or wondered if it had memories.
Drosophila sp. Different species of fruit flies are used as animal models.
We see terms like genes and genetics commonly nowadays, and sometimes what we hear about them sounds bizarre or even a little mystical. To put it simply, genes are bits of information stored in sequences of our DNA, comparable to books stored in a library or application programs stored in a computer. They give the information that makes the eyes brown or the hair yellow. Genetics is just the study of how that information is passed from one generation to the next. To make things more interesting, there are different levels of organization in which those pieces of information can be kept. DNA is packed in something called chromatin, and epigenetics is the study of how that packaging is stored. Just because someone has a particular gene, does not necessarily mean that the gene is accessible or functional. Just like your favourite book is at hand but that encyclopaedia from 1978 is somewhere in the attic, epigenetic modifications help the cell store away genes that are currently unnecessary, although they still exist in the DNA, they are inaccessible and non-functional.
Chromatin regulation is dynamic, keeping genes exposed or hiding them. Chromatin remodellers change the packaging of the collection of genes called the genome, opening or closing regions without changing the DNA sequence in the genes themselves. Just like a librarian moving books around.
Using different sources, researchers from the Kramer Lab found that a large number of genes altered in intellectual disability are related to the regulation of the chromatin and brain cell communication. They focused on the chromatin remodeller complex SWI/SNF (commonly pronounced Switch-Snif).
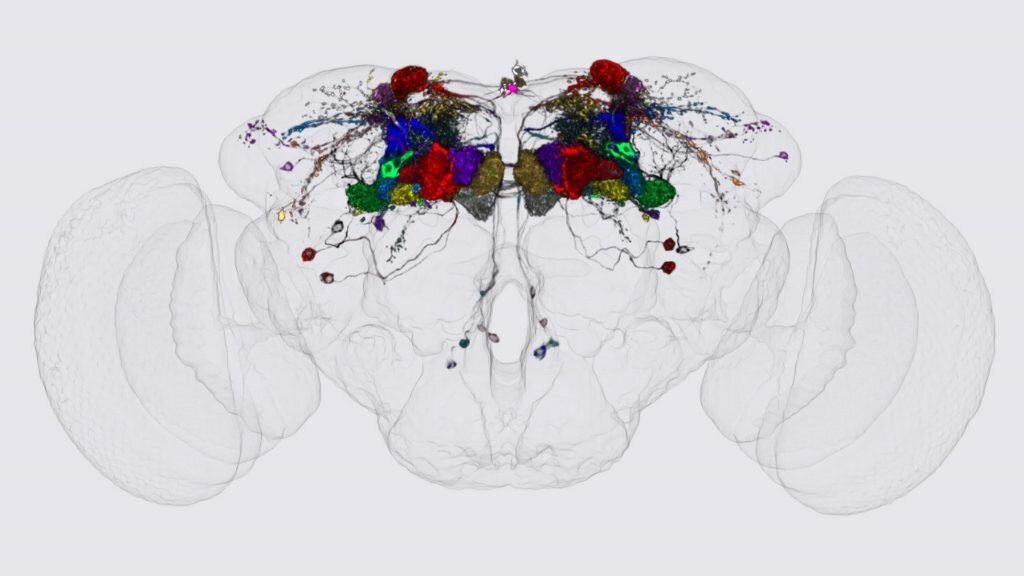
Researchers from the Kramer lab temporarily blocked the genes of different components of the SWI/SNF complex in the brains of fruit flies and saw the effects in brain development and function. They found that after blocking SWI/SNF, areas of the fly brain had defects, particularly one area called the mushroom body. In the picture, you can see the mushroom body inside the fly brain, the big blobs on the sides are the eyes, and the cells of the mushroom body are shown in different colours. Interestingly, this part of the brain is important for memory, and when they checked the memory of these abnormal flies, they indeed found memory impairments.
This sounds interesting and maybe exciting to some degree, but it’s in flies. Why flies? Why care about flies? Especially for something so complex like memory?
Fruit flies (the kind you see when you leave your bananas out for too long) are among the more traditional models used to study genes and heredity, they are a powerful tool for several reasons: a lot is known about their development, their genes can be very easily manipulated, they grow fast and in large numbers and we can reliably evaluate certain behaviours and even their memory. Of course, on many levels, flies and humans are completely different, but they have a surprising amount of gene homology (similarity). Astonishingly, around 75% of human genes linked to disease have a fly equivalent. There are equivalent versions of many mammalian genes in the fly, including chromatin remodellers, which are similar in structure and function.
Given the particular interest of the Kramer Lab, working on flies allowed them to manipulate genes at different developmental stages faster and far more easily than with other models, crossing and breeding is easier because flies develop rapidly and produce a lot of offspring. They found that when some SWI/SNF components are missing in different stages of development, cells in the brain called neurons fail to reshape as the brain matures, or even survive as the fly ages. For comparison, trying to study the same processes in other animals would take months or even years. Using the fly, they found evidence indicating that the SWI/SNF subunits have a potential role in neuronal development and degeneration.
Drosophila brain with the neurons of the mushroom body in different colours.
Having analysed how a defective SWI/SNF complex affects the brain, the Kramer lab wanted to test if these abnormalities had an effect on the memory of the fly. Amazingly, there are tests to evaluate fly memory. In one of these tests, a male and a female fly are put together, and if the female has already had a partner she will reject the male and he will eventually give up courtship. After being rejected, the male learns and courts the females less over time. However, if the male’s memory is deficient, they can’t remember their past failures and continue to attempt mating, only to be rejected over and over again. This is what happened in the Kramer lab’s experiment when components of SWI/SNF were missing in the male flies.
Many details regarding gene regulation in neurons remain unknown. This study by the Kramer lab shed light on the role of different components of the SWI/SNF complex in neuronal development and memory. Their work is a great example of how flies can be a powerful research tool, allowing scientists to modify single genes of interest and see the effects on brain development and behaviour. Further research will help improve our understanding of the mechanisms behind gene regulation in neurons that may be affected in intellectual disabilities. Next time I see Nick, I’ll gladly tell him that I’m a fly believer.
Original Research Article:
Chubak, M. C., Nixon, K., Stone, M. H., Raun, N., Rice, S. L., Sarikahya, M., … Kramer, J. M. (2019). Individual components of the SWI/SNF chromatin remodelling complex have distinct roles in memory neurons of the Drosophila mushroom body. Disease models & mechanisms, 12(3), dmm037325. doi:10.1242/dmm.037325